
Monochlorination Products Of Propane, Pentane, And Other Alkanes
How Many Monochlorination Isomers Are Formed From Free-Radical Chlorination Of Alkanes?
Last time we covered a comparatively simple reaction: free-radical chlorination of methane to (CH4) to give chloromethane (CH3Cl) and saw that the reaction proceeds through three stages - initiation (where free radicals are created), propagation (the main “product-forming” step of the chain reaction, where a chloroalkane is created without net formation of new free radicals) and termination (where radicals combine, resulting in a net reduction of the number of free radicals).
There’s one simple extension of this reaction I’d like to cover in this post. We just covered the simple molecule CH4. What happens when we move beyond CH4 to the monochlorination of more complex alkanes?
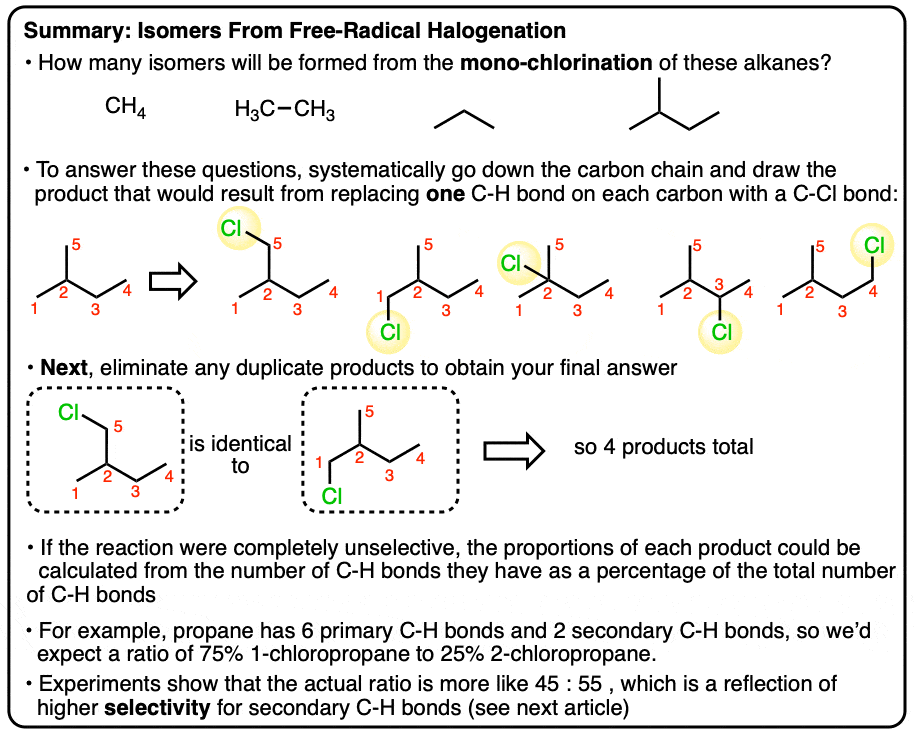
Table Of Contents
- Free-Radical Halogenation Of Methane And Ethane Can Only Give One Mono-Chlorinated Product
- How Many Mono-Chlorinated Isomers Are Formed From Propane?
- A More Complex Example: Monochlorination of Pentane
- The Case of 2-Methylpentane
- If Chlorination Were Completely Random, What Yields Of 1-Chloropropane and 2-Chloropropane Would We Expect To See?
- Test Yourself
- Notes
- (Advanced) References and Further Reading
1. Free Radical Halogenation Of Methane And Ethane Can Give Only One Monochlorinated Product
In the last post we discussed mono-chlorination of methane. It’s easy to talk about this reaction since all the hydrogens are equal. No matter which hydrogen you replace, you get the same product (CH3Cl).
Likewise, mono-chlorination of ethane also gives just one product: chloroethane.
(We can say that the hydrogens in methane and ethane are “homotopic“, but that’s a discussion for another day).
What about more complicated cases, like propane, butane, pentane, or even 2-methylpentane?
2. How Many Monochlorinated Isomers Are Formed From Propane?
Let’s start with propane. The first step here, if it isn’t immediately obvious, is to be aware of the “hidden” or “implicit” hydrogens on the line diagrams shown above - it’s important to be able to expand out a line diagram to a condensed formula.
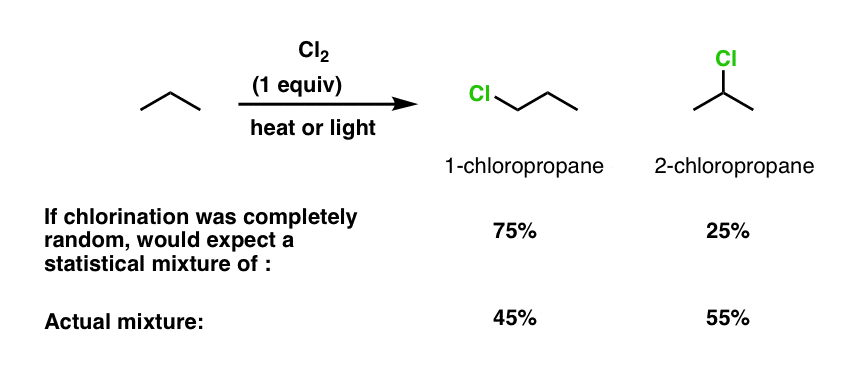
The next step is to go about drawing the various possibilities as we replace a single hydrogen on propane with chlorine. For propane, hopefully it should be clear that there are only two possiblities: chlorination at C-1 or C-2 (chlorination at C-3 would give the same product as that at C-1).
These two products (1-chloropropane and 2-chloropropane) are constitutional isomers.
[Question to think about: If the chlorination of propane was completely random, what yields of 1-chloropropane and 2-chloropropane would you expect to see? Answer below]
3. A More Complex Example: Monochlorination of Pentane
Similarly, what do we get for the slightly more complicated example of pentane? There’s no mathematical formula for figuring this out, but as with many things in organic chemistry, it can pay dividends to be systematic. Start at one end and work towards the other. One thing that can help is applying IUPAC nomenclature to each possibility - it can assist in realizing if you’ve drawn a duplicate.
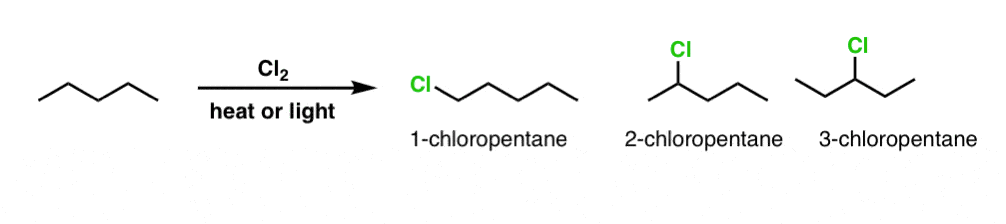
We have 3 possibilities for constitutional isomers: 1-chloropentane, 2-chloropentane, and 3-chloropentane.
[note that I said “constitutional isomers” - can you see possibilities for stereoisomers in any of these molecules? [Note 1]
4. Monochlorination Of 2-Methylpentane
Finally let’s look at another slightly more complex example: 2-methylpentane.
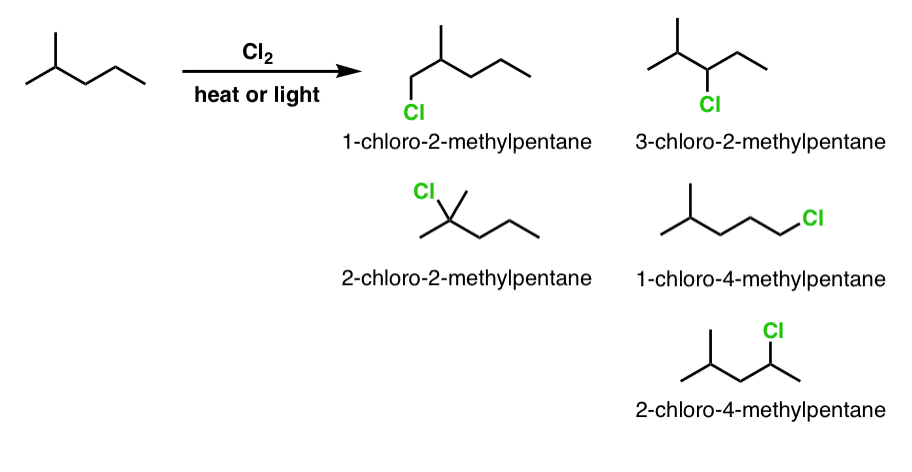
Here, we have 5 constitutional isomers possible (not counting stereoisomers). Again, it helps to break out your IUPAC nomenclature to double-check that there are no duplicates.
It is impossible to capture the variety of potential questions with these three examples, but the general thrust is the same. It also helps to have a systematic approach - starting the exchange of hydrogen for chlorine at one side of the molecule, and gradually working to the other side.
5. If Chlorination Was Completely Random, What Yields Of 1-Chloropropane and 2-Chloropropane Would We Expect To See?
Question answer: If the chlorination of propane was completely random, what yields of 1-propane and 2-propane would you expect to see?
Well, there’s 6 methyl hydrogens, and 2 “methylene” (CH2) hydrogens. So you’d expect to see a ratio of 75% 1-chloropropane to 25% 2-chloropropane.
Is that what’s observed? No!
Instead, experiments show that free-radical chlorination of propane in the gas phase at 25°C give 45% 1-chloropropane to 55% 2-chloropropane!
Why that might be? We’ll talk about that in the next post.
6. Test Yourself on Monochlorination of Alkanes
Here’s some more practice problems. How many constitutional isomers would you expect to see for the mono-chlorination of each of these molecules?
Next Post: Selectivity In Free Radical Reactions
Notes
Note 1. Hopefully you can see that 2-chloropentane has a chiral center, so can exist as either (R)-2-chloropentane or (S)-2-chloropentane. Under free radical conditions we will obtain a racemic mixture of these two compounds (i.e. 50% mixture of (R) and (S).
(Advanced) References and Further Reading
- Syntheses from Natural Gas Hydrocarbons Identity of Monochlorides from Chlorination of Simpler Paraffins B. Hass, E. T. McBee, and Paul Weber Industrial & Engineering Chemistry 1935, 27 (10), 1190-1195 DOI: 10.1021/ie50310a025 The source of the 41:59 ratio of 1-chloropropane to 2-chloropropane for the chlorination of propane is from this 1935 paper, the first in a series of rigorous studies on the halogenation of simple alkanes (paraffins) under both free-radical and thermal conditions. One notable advance of Hass’ work here was in obtaining pure standards for each of the monochlorination products, so that their physical properties could be measured and the yields of the reactions accurately reported.
- Chlorination of Paraffins B. Hass, E. T. McBee, and Paul Weber Industrial & Engineering Chemistry 1936, 28 (3), 333-339 DOI: 10.1021/ie50315a017 The above two papers contain what is now known as “Hass’ rules for radical chlorination”. The first five of these rules are summarized below: 1. If the temperature is controlled to prevent pyrolysis, no skeletal rearrangements will occur, and every possible monochloride will form. 2. The rate of substitution of hydrogen atoms is in the order tertiary > secondary > primary 3. As temperature increases, selectivity of chlorination decreases (it approaches 1:1:1 (tertiary:secondary:primary)) 4. Liquid phase chlorination is less selective than gas-phase chlorination 5. Once initiated, chlorination is unaffected by moisture, light, and ‘carbon surfaces’ Pyrolysis is the decomposition of organic materials at high temperatures, usually at 430 °C or higher. The reason for Rule #4 is that the reaction is 2nd order and is dependent on the concentration of both methane and chlorine. The liquid phase, being a condensed phase, is much more concentrated than the gas phase.The paper also calculates a relative reaction rate of 4.43 : 3.25 : 1.00 for the chlorination of tertiary, secondary, and primary C-H bonds, respectively, for a reaction at 300°C in the vapor phase.
- Chlorination of Methane T. McBee, H. B. Hass, C. M. Neher, and H. Strickland Industrial & Engineering Chemistry 1942, 34 (3), 296-300 DOI: 10.1021/ie50387a009 This paper shows the design of a reactor for the chlorination of methane such that the reaction can be controlled to give any of the desired chloromethanes in high yield. This is of significance because CH3Cl, CH2Cl2, CHCl3, and CCl4 are all important feedstocks or solvents and this is how they are produced industrially.
- KINETICS OF THE THERMAL CHLORINATION OF METHANE Robert N. Pease and George F. Walz Journal of the American Chemical Society 1931, 53 (10), 3728-3737 DOI: 10.1021/ja01361a016 This paper provides kinetic evidence that chlorination of methane is 2nd order (first order in both methane and Cl2).
Link nội dung: https://loptienganh.edu.vn/pentan-cl2-a74856.html